Milk thistle for treatment of nonalcoholic fatty liver disease
Authors:
Abenavoli L
Department of Experimental and Clinical Medicine, University of Magna Græcia, Catanzaro, Italy
Aviello G
Department of Experimental Pharmacology, University of Federico, Naples, Italy
Capasso R
Department of Experimental Pharmacology, University of Federico, Naples, Italy
Milic N
Department of Pharmacy, University of Novi Sad, Novi Sad, Serbia
Capasso F
Department of Experimental Pharmacology, University of Federico, Naples, Italy
Correspondence:
Ludovico Abenavoli,
Department: Department of Experimental and Clinical Medicine, University of Magna Græcia
Address: Department of Experimental and Clinical Medicine, University of Magna Græcia, Viale Europa
City: Catanzaro
Country: Italy
E-mail: l.abenavoli@unicz.it
Tel: +39-9613697113
Fax: +39-961754220
Abstract:
Nonalcoholic fatty liver disease (NAFLD) is one the most common causes of chronic liver disorders in the Western world. These patients have many significant comorbidities. The therapeutic approach to NAFLD is based on lifestyle intervention, but there is no consensus on the ideal pharmacological treatment. Silybum marianum, commonly known as milk thistle (MT), is one of the oldest and most extensively researched plants in the treatment of liver diseases. Many studies have demonstrated that the active components of MT silymarin have many hepatoprotective properties. In recent years, several preclinical and clinical reports have described the efficacy of silymarin as a treatment for NAFLD. The chief aim of this review is to discuss the newest and most promising applications of MT in the treatment of NAFLD.
Keywords: Milk thistle; Silymarin; Nonalcoholic fatty liver disease; Fibrosis; Insulin resistance
--------------------------------------------------------------------------------
Implication for health policy/practice/research/medical education:
This article describes the importance of natural treatment regimen like plant extracts in treating NAFLD and can be attended by general practitioners and family physicians and others who are involved in treating patients with liver disorders.
Please cite this paper as:
Abenavoli L, Aviello G, Capasso R, Milic N, Capasso F. Milk thistle for treatment of nonalcoholic fatty liver disease. Hepat Mon. 2011;11(3):173-177.
Article history:
Received: 25 Sep 2010
Revised: 14 Jan 2011
Accepted: 17 Jan 2011
2011 Kowsar M.P.Co. All rights reserved.
Manuscript:
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one the most common causes of chronic liver disorders in the Western world. These patients have many significant comorbidities (e.g., diabetes, hypothyroidism and metabolic syndrome) (1). Its incidence in adults and children is rising rapidly due to the current obesity and type 2 diabetes epidemics (2). It is a multifaceted metabolic disorder and is encountered in clinical practice by many health care specialists-from primary care physicians and gastroenterologists to cardiologists, radiologists, and gynecologists. The umbrella term "NAFLD" encompasses simple steatosis, nonalcoholic steatohepatitis (NASH), and advanced fibrosis or cirrhosis that is related to this pathological entity (3). The mechanism of the occurrence and progression of the underlying steatosis to liver disease is poorly understood but is likely driven by several factors that are expressed in the context of genetic predisposition. In this complex repertoire, a two-step hypothesis has been proposed, in which the first step induces the accumulation of liver fat and the second step effects the progression of steatosis to NASH (4, 5).
Obesity, insulin resistance, oxidative stress, and cytokine and adipokines mediate the pathogenesis of NAFLD. These factors can promote and enhance inflammation, cell injury, apoptosis, fibrinogenesis, and carcinogenesis, leading to the accumulation of fat, reflecting the development and progression of the disease. With regard to therapy, the approach to NAFLD is based on lifestyle intervention, and there is no consensus on the ideal pharmacological treatment (6). Accordingly, weight reduction, regular physical activity, and insulin-sensitizing drugs have been used widely and examined in several studies. Other treatment approaches include the consumption of special diets, antioxidants, and cytoprotective therapy.
Silybum marianum, commonly known as milk thistle (MT) (family Asteraceae/Compositae) is one of the oldest and most extensively studied plants in the treatment of liver diseases. This plant grows as a stout thistle in rocky soils, generating large purple flowering heads. Its leaves are characterized by milky veins, from which the plant derives its name (7). MT was used by ancient physicians and herbalists to treat liver and gallbladder disorders, including hepatitis, cirrhosis, and jaundice, and to protect the liver against poisoning from chemical and environmental toxins, including snake bites, insect stings, mushroom poisoning, and alcohol. The active complex of MT is a lipophilic extract from its seeds and comprises three flavonolignan isomers, collectively known as silymarin. Silymarin acts as an antioxidant by reducing free radical production and lipid peroxidation and has antifibrotic activity, limiting the activation of hepatic stellate cells, inducing hepatic stellate cell apoptosis, and evoking the degradation of collagen deposits (8). In addition, the ameliorative effects of silymarin in NAFLD patients might be attributed to its activity against glucose and lipid metabolism. Silymarin inhibits the activation of NF-kB and its related pathways in the liver. The principal aim of this review is to identify the newest and most promising applications of MT in the treatment of NAFLD.
Biochemistry and pharmacology of milk thistle
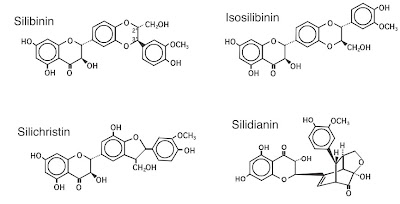
The active extract of MT, known as silymarin, is a mixture of flavanolignans (Figure 1): silibinin, isosilibin, silidianin, and silichristine. Silymarin is extracted from dried MT seeds, in which it exists in higher concentrations than in other parts of the plant. The structural similarity of silymarin to steroid hormones is believed to mediate its protein synthesis facilitatory actions. Silibin is the predominant and most active component, constituting approximately 60% to 70% of the isomers, followed by silichristin (20%), and silidianin (10%) (7, 8). Most of its hepatoprotective properties are attributed to silybin (silibinin), which is the chief constituent (60% to 70%) of silymarin (7, 8). Silymarin constitutes at least 70% of standardized milk thistle. It can be extracted with aqueous alcohol (95%) as a rich, bright yellow fraction. A hydroextraction technique has also been developed to extract silymarin from MT (9). The silymarin content in milk thistle extracts can vary from 40% to 80% (8). The drug can be examined with regard to its microscopic characteristics by thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), and spectrophotometry (10, 11).
Figure 1. Structures of the components of silymarin.
Silymarin is insoluble in water and is typically administered as a sugar-coated tablet or an encapsulated standardized extract. Approximately 20% to 50% of silymarin is absorbed following oral administration in humans, and roughly 80% of the dose is excreted in bile, while about 10% enters enterohepatic circulation (11). Pharmacokinetic studies, however, have been performed primarily using silibinin. The bioavailability of silibinin is low and appears to depend on several factors, such as (i) the content of accompanying substances that have solubilizing properties, such as other flavonoids, phenol derivates, amino acids, proteins, tocopherol, fat, cholesterol, and other substances that are found in the preparation; and (ii) the concentration of the preparation itself (12). The systemic bioavailability can be enhanced by adding solubilizers to the extract (13).
The bioavailability of silybinin can also be increased by complexation with phosphatidylcholine or ß-cyclodextrin and, possibly, by the choice of the capsule material (14). Pharmacokinetic studies on the silybin-phosphatidylcholine complex have demonstrated increased oral bioavailability of silybin in healthy human subjects, likely due to facilitation of the passage of the drug across the gastrointestinal tract by the drug complex (15). The variations in the content, dissolution, and oral bioavailability of silybinin between commercially available silymarin-containing products (despite the same declaration of content) are significant (16). Therefore, comparisons between studies should be made with caution, based on the analytical method (TLC vs. HPLC) and whether free, conjugated, or total silybinin is being measured. Systemic plasma concentrations are usually measured-although silymarin is active in the liver-because they are an estimate of the quantity of the drug that is absorbed from the gastrointestinal tract. The adequate bioavailability accounts for the dose-related oral activity of silymarin in the liver (13-17).
In male volunteers, after a single administration of a standard dose of oral silibinin 100 to 360 mg, the Cmax of plasma silibinin was reached after approximately 2 hours and ranged between 200 and 1400 μg/L, of which approximately 75% was present in conjugated form (15, 16, 18). The elimination half-life of total silibinin was approximately 6 hours (19, 20). Between 3% and 8% of the oral dose was excreted in the urine, and 20% to 40% was recovered from the bile as glucuronide and sulfate conjugates. The remainder was excreted in feces. Silibinin concentrations in the bile were approximately 100-fold higher than in the serum (10-5 to 10-4 mol/L of silibinin in bile), with concentrations peaking within 2 to 9 hours (19). At oral doses of 20 g/kg in mice and 1 g/kg in dogs, silymarin effects low toxicity and no mortality or adverse effects. After intravenous infusion, its LD50 was 400 mg/kg in mice, 385 mg/kg in rats, and 140 mg/kg in rabbits and dogs (21). Although silymarin has a good safety record, there are several reports of gastrointestinal disturbances and allergic skin rashes with its use (22). These data demonstrate that the acute, subacute, and chronic toxicity of silymarin is very low.
Hepatoprotective effects of milk thistle
The active extract has antioxidant, anti-inflammatory, and antifibrotic properties; in addition, it stimulates protein biosynthesis and liver regeneration. There are four overarching hepatoprotective activities of silymarin: (i) its effects against lipid peroxidation due to free radical scavenging and the ability to increase the cellular content of glutathione (GSH); (ii) its ability to regulate membrane permeability and increase membrane stability in the presence of xenobiotic damage; (iii) its capacity to regulate nuclear expression through steroid-like effects; and (iv) its inhibition of the transformation of stellate hepatocytes into myofibroblasts, which mediate the deposition of collagen fibers, leading to cirrhosis (23-26). In addition, MT inhibits the absorption of toxins, such as phalloidin and α-amanitin, preventing them from binding to the cell surface and inhibiting membrane transport systems. Further, by interacting with the lipid component of cell membranes, silymarin and silibinin can modulate their chemical and physical properties. They stabilize the membranes of hepatocytes and thus prevent toxins from entering them from enterohepatic circulation. They promote liver regeneration by stimulating nucleolar polymerase A and increasing ribosomal protein synthesis (27). Silymarin inhibits the expression of adhesion molecules, such as E-selectin, another family of transmembrane molecules, which are expressed preferentially on the surface of leukocytes (28). Its hepatoprotective properties against a wide range of liver damage-inducing agents render MT a unique drug.
Milk thistle in liver steatosis
Several well-designed experimental studies have suggested that silymarin exerts beneficial effects in chronic liver diseases, particularly in NAFLD (Figure 2). For example, silymarin interferes with leukotriene formation in Kupffer cell (KC) cultures, thus inhibiting hepatic stellate cell (HSC) activation, a crucial event in fibrogenesis (26). In addition, 10-4 mol/l silymarin blocks the proliferation of HSC cultures and their transformation into myofibroblasts (29). Velussi et al. (30) studied the efficacy of silymarin in reducing lipid peroxidation and insulin resistance in diabetic patients with alcoholic cirrhosis. The study was performed in alcoholic cirrhosis patients, who have similar natural histories and pathological features as alcoholic liver disease and NASH patients. In this randomized, controlled, unblinded, 12-month study, one group (n = 30) received 600 mg silymarin per day plus standard therapy, and the control group (n = 30) received standard therapy alone. The efficacy parameters, measured regularly throughout the study, included fasting blood glucose levels; mean daily blood glucose levels, daily glycosuria levels, glycosylated hemoglobin (HbA1c), and malondialdehyde (MDA) levels, a marker of lipid peroxidation
Figure 2. Pathogenic mechanisms in the histological progression of NAFLD and the site of action of sylimarin (crossed circle) (CYP2E1: cytochrome P450 2E1, ROS: reactive oxygen species, HSCs: hepatic stellate cells, KC: Kupffer cells)
There was a significant decrease (p < 0.01) in fasting blood glucose levels, mean daily blood glucose levels, daily glycosuria, and HbA1c levels after 4 months of treatment with silymarin. Moreover, fasting insulin levels and mean exogenous insulin requirements declined in the treated group (p < 0.01), and the control group experienced an increase (p < 0.05) in fasting insulin levels and stabilized their need for insulin. These findings were consistent with the significant decrease (p ; 0.01) in basal and glucagon-stimulated C-peptide levels in the treated group and the rise in both parameters in the control group. Notably, MDA levels fell in the treated group (p < 0.01). These studies demonstrate that treatment with silymarin reduces lipoperoxidation of cell membranes and insulin resistance, decreasing the overproduction of endogenous insulin and the need for exogenous insulin significantly.
Subsequently, Loguercio et al. (31) evaluated the antioxidant and antifibrotic activity of a complex that comprised silybin, vitamin E, and phospholipids (Realsil ® IBI-Lorenzini Pharmaceutical, Italy) against insulin resistance and liver damage in patients with NAFLD and chronic HCV infection. This study enrolled 85 patients; 59 were affected by primitive NAFLD (group A), and 26 had HCV-related chronic hepatitis C with NAFLD, all HCV genotype-1b, and non-responders to the previous antiviral treatment (group B). All patients with a diagnosed liver disease in the 2 years prior to the study, based on histological criteria, were enrolled over 6 consecutive months and subdivided using a systematic random sampling procedure: 53 patients (39 NAFLD and 14 HCV) were treated with 4 tablets/day of Realsil ® (one tablet contained 94 mg of silybin, 194 mg of phosphatidylcholine, and 90 mg of vitamin E) for 6 months, followed by another 6 months of follow-up, and 32 patients (20 NAFLD and 12 HCV) constituted the control group (no treatment).
At 0, 6, and 12 months, the following outcomes were measured: body mass index (BMI), bright liver by ultrasonography (US), transaminase and GGT levels, blood glucose and insulin plasma levels with simultaneous measurement of insulin resistance by Homeostasis Model Assessment (HOMA) test, and plasma levels of transforming growth factor ß, hyaluronic acid and metalloproteinase as indices of liver fibrosis. Group A showed a significant and persistent reduction in US score for liver steatosis that ranged p < 0.01. Plasma levels of liver enzymes fell in treated patients but not in the control group, but this effect lasted only in NAFLD patients. Hyperinsulinemia, present in both groups, declined only in treated patients (p < 0.005). Realsil ® significantly reduced all indices of liver fibrosis in both treatment groups, persisting only in group B.
In a randomized clinical trial, Hajaghamohammadi et al. (32) examined the efficacy of silymarin in 50 NAFLD patients. The study population, comprising 32 men (64%) and 18 women (36%), was divided into case and control groups. All patients had elevated liver enzymes and increased liver echogenicity by US. The case group was treated with one tablet that contained 140 mg silymarin per day for 2 months; the control group was treated similarly with placebo. Before and after the study, weight, BMI, and liver transaminase levels were measured for each patient. The authors did not observe any significant differences in mean weight or BMI before or after the study in either group. In the case group, mean alkaline transaminase (ALT) and aspartate transaminase (AST) levels deceased from 103.1 to 41.4 U/L and 53.7 to 29.1 IU/ml, respectively (p < 0.001 and p < 0.001, respectively). In the control group, the decreases in mean ALT and AST (7.8 and 2.2 IU/ml respectively) were not significant.
The effect of silymarin on transaminase levels was confirmed by another Iranian study (33). One hundred subjects with NASH were randomized into two groups: group A, comprising 29 males and 21 females, received placebo, and group B, with 28 males and 22 females, received 280 mg silymarin for 6 months. The mean serum ALT level in the silymarin group was 113.03 and 73.14 IU/ml before and after the treatment, respectively (p = 0.001). ALT normalization (ALT < 40) was observed in 18% and 52% of patients in groups A and B, respectively (p = 0.001). AST normalization (AST < 40) was observed in 20% of cases in the placebo group and in 62% of cases in the silymarin-treated group (p = 0.0001). Mitochondria regulate hepatocyte metabolism, constituting the site of ß-oxidation and oxidative phosphorylation. Oxidative stress in NASH is closely related to mitochondrial dysfunction (34). During the progression of NASH, the excess of free fatty acids increases mitochondrial H²O² production, which in turn oxidizes mitochondrial membranes and regulates the activity of uncoupling protein 2 (UCP2) and carnitine palmitoyl transferase-1 (CPT-1) (35).
Serviddio et al. (36) examined the effects of the silybin-phospholipid complex on liver redox balance and mitochondrial function in a dietary model of NASH, measuring glutathione oxidation, mitochondrial oxygen uptake, proton leak, ATP homeostasis, and H2O2 production rate in liver mitochondria from rats that were fed a methionine/choline-deficient diet (MCD) and MCD plus SILIPHOS for 7 and 14 weeks. Oxidative proteins, hydroxynonenal (HNE) - and MDA-protein adducts, and mitochondrial membrane lipid composition were also assessed. SILIPHOS limited glutathione depletion and mitochondrial H2O2 production. Moreover, this complex preserved mitochondrial bioenergetics and prevented mitochondrial proton leakage and ATP reduction. The silybin-phospholipid complex limited the formation of HNE- and MDA-protein adducts. In conclusion, this complex prevents severe oxidative stress and preserves hepatic mitochondrial bioenergetics in MCD-induced NASH. The alterations in mitochondrial membrane fatty acid composition that were induced by the MCD diet were prevented in part by silybin and phospholipids, which conferred anti-inflammatory and antifibrotic effects.
Recently Haddad et al. (37) examined the therapeutic effect of silibinin in an experimental rat model of NASH. The control group was fed a standard liquid diet for 12 weeks, and the test animals were fed a high-fat liquid diet for 12 weeks with or without (NASH) a daily supplement of silibinin-phosphatidylcholine complex (silibinin 200 mg/kg) for the last 5 weeks. The NASH rats developed all hallmarks of the pathology. Treatment with silibinin improved liver steatosis and inflammation and decreased lipid peroxidation, plasma insulin, and TNF-alpha (p<0.05). In addition, silibinin decreased the release of free radicals and restored relative liver weights and GSH levels (p<0.05). The authors concluded that a complex with phosphatidyl-choline is effective in reversing steatosis, inflammation, oxidative stress, and insulin resistance in an in vivo rat model of diet-induced NASH.
Conclusions
NAFLD and its various stages affect much of the world's population. The pathogenic mechanisms of liver damage that are involved in NAFLD are complicated and comprise a series of sequential steps. With regard to therapy, the approach to NAFLD is currently based on lifestyle intervention, but there is no consensus on the ideal pharmacological treatment (2). The drugs that are used to treat NAFLD should reduce body weight, improve insulin resistance and other metabolic alterations, reduce the link between adipose tissue and liver function by acting as anti-inflammatory and immunomodulatory agents, and modulate the progression of liver steatosis to inflammation and fibrosis by blocking oxidative stress. A multifaceted approach to NAFLD, entailing several treatment options, is likely to be developed soon. Among these strategies, the use of complementary and alternative medicines, such as natural antioxidants and hepatoprotective plant products, has been widely accepted in the past decade. Silymarin is one of the most successful examples of a modern drug that arose from traditional healing practices. It is favored in treating various liver diseases due to its oral efficacy, good safety profile, and, most importantly, affordability.
Several pharmacological studies have been performed on the active components of MT, silymarin, and silibinin. These substances have hepatoprotective, antioxidant, anti-inflammatory, and antifibrotic properties; in addition, they stimulate protein biosynthesis and liver regeneration and have immunomodulatory activity (7). Particularly with regard to NAFLD patients, the ameliorative effects of silybin in diabetic patients, due to improved insulin activity, reductions in lipid peroxidation, and restoration of GSH levels, might explain its efficacy against liver steatosis (31, 38). Based on the literature, we believe that MT is a useful medicinal herb that is a viable therapeutic option for treating patients with NAFLD.
Finantial support
None declared.
Conflicts of Interest
The authors have declared that there is no conflict of interest.
References:
1. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221-31. [PubMed]
2. Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49(1):306-17. []
3. Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123(5):1705-25 [Link]
4. Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917-23. [PubMed]
5. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-73. [PubMed]
6. Petta S, Muratore C, Craxi A. Non-alcoholic fatty liver disease pathogenesis: the present and the future. Dig Liver Dis. 2009;41(9):615-25. [PubMed]
7. Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24(10):1423-32. [PubMed]
8. Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124(5):491-504. [PubMed]
9. Duan L, Carrier DJ, Clausen EC. Silymarin extraction from milk thistle using hot water. Appl Biochem Biotechnol. 2004;114(1-3):559-68. [PubMed]
10. Kvasnicka F, Biba B, Sevcik R, Voldrich M, Kratka J. Analysis of the active components of silymarin. J Chromatogr A. 2003;990(1-2):239-45. [PubMed]
11. Lee JI, Hsu BH, Wu D, Barrett JS. Separation and characterization of silybin, isosilybin, silydianin and silychristin in milk thistle extract by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2006;1116(1-2):57-68. [PubMed]
12. Voinovich D, Perissutti B, Grassi M, Passerini N, Bigotto A. Solid state mechanochemical activation of Silybum marianum dry extract with betacyclodextrins: Characterization and bioavailability of the coground systems. J Pharm Sci. 2009;98(11):4119-29. [PubMed]
13. Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035-63. [PubMed]
14. Morazzoni P, Bombardelli E. Silybum marianum (Carduus marianus). Fitoterapia. 1995;66(1):3-42. [Link]
15. Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E. Pharmacokinetic studies on IdB 1016, a silybin- phosphatidylcholine complex, in healthy human subjects. Eur J Drug Metab Pharmacokinet. 1990;15(4):333-8. [PubMed]
16. Schulz HU, Schurer M, Krumbiegel G, Wachter W, Weyhenmeyer R, Seidel G. [The solubility and bioequivalence of silymarin preparations]. Arzneimittelforschung. 1995;45(1):61-4. [PubMed]
17. Gatti G, Perucca E. Plasma concentrations of free and conjugated silybin after oral intake of a silybin-phosphatidylcholine complex (silipide) in healthy volunteers. Int J Clin Pharmacol Ther. 1994;32(11):614-7. [PubMed]
18. Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Investig. 2002;22(1):51-65. [Link]
19. Yu JN, Zhu Y, Wang L, et al. Enhancement of oral bioavailability of the poorly water-soluble drug silybin by sodium cholate/phospholipid-mixed micelles. Acta Pharmacol Sin. 2010;31(6):759-64. [PubMed]
20. Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15(1):9-20. [PubMed]
21. An adverse reaction to the herbal medication milk thistle (Silybum marianum). Adverse Drug Reactions Advisory Committee. Med J Aust. 1999;170(5):218-9. [PubMed]
22. Comelli MC, Mengs U, Schneider C, Prosdocimi M. Toward the definition of the mechanism of action of silymarin: activities related to cellular protection from toxic damage induced by chemotherapy. Integr Cancer Ther. 2007;6(2):120-9. [PubMed]
23. Polyak SJ, Morishima C, Lohmann V, et al. Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci USA. 2010;107(13):5995-9. [PubMed]
24. Kiruthiga PV, Shafreen RB, Pandian SK, Devi KP. Silymarin protection against major reactive oxygen species released by environmental toxins: exogenous H2O2 exposure in erythrocytes. Basic Clin Pharmacol Toxicol. 2007;100(6):414-9. [PubMed]
25. Post-White J, Ladas EJ, Kelly KM. Advances in the use of milk thistle (Silybum marianum). Integr Cancer Ther. 2007;6(2):104-9. [PubMed]
26. Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23(4):749-54. [PubMed]
27. Basiglio CL, Sanchez Pozzi EJ, Mottino AD, Roma MG. Differential effects of silymarin and its active component silibinin on plasma membrane stability and hepatocellular lysis. Chem Biol Interact. 2009;179(2-3):297-303. [PubMed]
28. Kang JS, Park SK, Yang KH, Kim HM. Silymarin inhibits TNF-alpha-induced expression of adhesion molecules in human umbilical vein endothelial cells. FEBS Lett. 2003;550(1-3):89-93. [PubMed]
29. Fuchs EC, Weyhenmeyer R, Weiner OH. Effects of silibinin and of a synthetic analogue on isolated rat hepatic stellate cells and myofibroblasts. Arzneimittelforschung. 1997;47(12):1383-7. [PubMed]
30. Velussi M, Cernigoi AM, De Monte A, Dapas F, Caffau C, Zilli M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26(4):871-9. [PubMed]
31. Loguercio C, Federico A, Trappoliere M, et al. The effect of a silybin-vitamin e-phospholipid complex on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52(9):2387-95. [PubMed]
32. Hajaghamohammadi AA, Ziaee A, Rafiei R. The Efficacy of Silymarin in Decreasing Transaminase Activities in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. Hepat Mon. 2008;8(3):191-5. [Hepat Mon]
33. Hashemi SJ, Hajiani E, Sardabi EH. A Placebo-Controlled Trial of Silymarin in Patients with Nonalcoholic Fatty Liver Disease. Hepat Mon. 2009;9(4):265-70. [Hepat Mon]
34. Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40(4):578-84. [PubMed]
35. Serviddio G, Bellanti F, Tamborra R, et al. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut. 2008;57(7):957-65. [PubMed]
36. Serviddio G, Bellanti F, Giudetti AM, et al. A silybin-phospholipid complex prevents mitochondrial dysfunction in a rodent model of nonalcoholic steatohepatitis. J Pharmacol Exp Ther. 2010;332(3):922-32. [PubMed]
37. Haddad Y, Vallerand D, Brault A, Haddad PS. Antioxidant and Hepatoprotective Effects of Silibinin in a Rat Model of Nonalcoholic Steatohepatitis. Evid Based Complement Alternat Med. 2011;[Epub ahead of print]. [PubMed]
38. Lirussi F, Beccarello A, Zanette G, et al. Silybin-beta-cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti-oxidant agent. Diabetes Nutr Metab. 2002;15(4):222-31. [PubMed]
This blog is all about current FDA approved drugs to treat the hepatitis C virus (HCV) with a focus on treating HCV according to genotype, using information extracted from peer-reviewed journals, liver meetings/conferences, and interactive learning activities.
Risk Of Developing Liver Cancer After HCV Treatment
- Home
- Newly Diagnosed With Hep C? Or Considering Treatment?
- All FDA Approved Drugs To Treat Hepatitis C
- Hepatitis C Genotypes and Treatment
- Mavyret (glecaprevir/pibrentasvir)
- Vosevi (Sofosbuvir/Velpatasvir/Voxilaprevir)
- Epclusa® (Sofosbuvir/Velpatasvir)
- Harvoni® (Ledipasvir/Sofosbuvir)
- VIEKIRA XR/VIEKIRA Pak
- Zepatier(Elbasvir/Grazoprevir)
- Cure - Achieving sustained virologic response (SVR) in hepatitis C
- HCV Liver Fibrosis
- FibroScan® Understanding The Results
- HCV Cirrhosis
- Staging Cirrhosis
- HCV Liver Cancer
- Risk Of Developing Liver Cancer After HCV Treatment
- Treating Elderly HCV Patients
- Fatty Liver Disease: NAFLD/NASH
- Current research articles on ailments that may be related to HCV
- Is There A Natural Way To Improve Liver Fibrosis?
- Can Food Or Herbs Interact With Conventional Medical Treatments?
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment